What You Should Know About the Mentor Implants Recall
You may or may not have read about Allergan’s decision to voluntarily recall all inventory of BIOCELL®textured breast implants and tissue expanders. There’s been a lot of buzz about the news, and some of the headlines out there can understandably be alarming.
Here’s a little more information to help you understand why their textured breast implant were removed from the market after nationwide distribution and what you should know about the recall, especially if you feel that this announcement may personally affect you.
FIRST THINGS FIRST, WHAT EXACTLY IS BIA-ALCL?
The reason the FDA determined that these textured breast implant are no longer sold or used is because a link has been found between textured implants and a rare type of lymphoma called Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). BIA-ALCL isn’t breast cancer, but a lymphoproliferative disorder classified as a slow growing type of non-Hodgkin’s lymphoma, or a disorder of the immune system.
Each year the Aesthetic Surgery Education and Research Foundation (ASERF) and similar organizations are funding research projects to further understand what exactly is causing BIA-ALCL to form in some women, but not in others. Current theories support a bacteria mediated lymphatic disorder. This happens in textured breast implant because there is an increased surface area in which bacteria are able to remain protected from the body’s immune system. Over time, this results in chronic inflammation and the development of abnormal immune cells.
WHY WERE THESE IMPLANTS SPECIFICALLY RECALLED?
There are many types of breast implants on the market which are currently approved by the FDA for use. Within the United States, all breast implants are manufactured by one of four companies: Allergan, Mentor, Sientra, and Ideal. Only Allergan, Mentor, and Sientra manufacture implants with surface texturing. It’s very important to understand that the FDA recall only applies to Allergan’s BIOCELL® TEXTURED breast implants and expanders.
So why was only one type of breast implant from one company singled out? As of July 6, 2019, the FDA has recorded 573 cases of BIA-ALCL. Of these cases, 481 have been attributed to Allergan’s Biocell texturing. Of those 573 cases, there have been 33 deaths as a result of a late or delayed diagnosis of BIA-ALCL.
In evaluating all cases of BIA-ALCL, not a single case to date has been discovered in women who have only had smooth breast implants, but is associated with breast implant which have texturing.
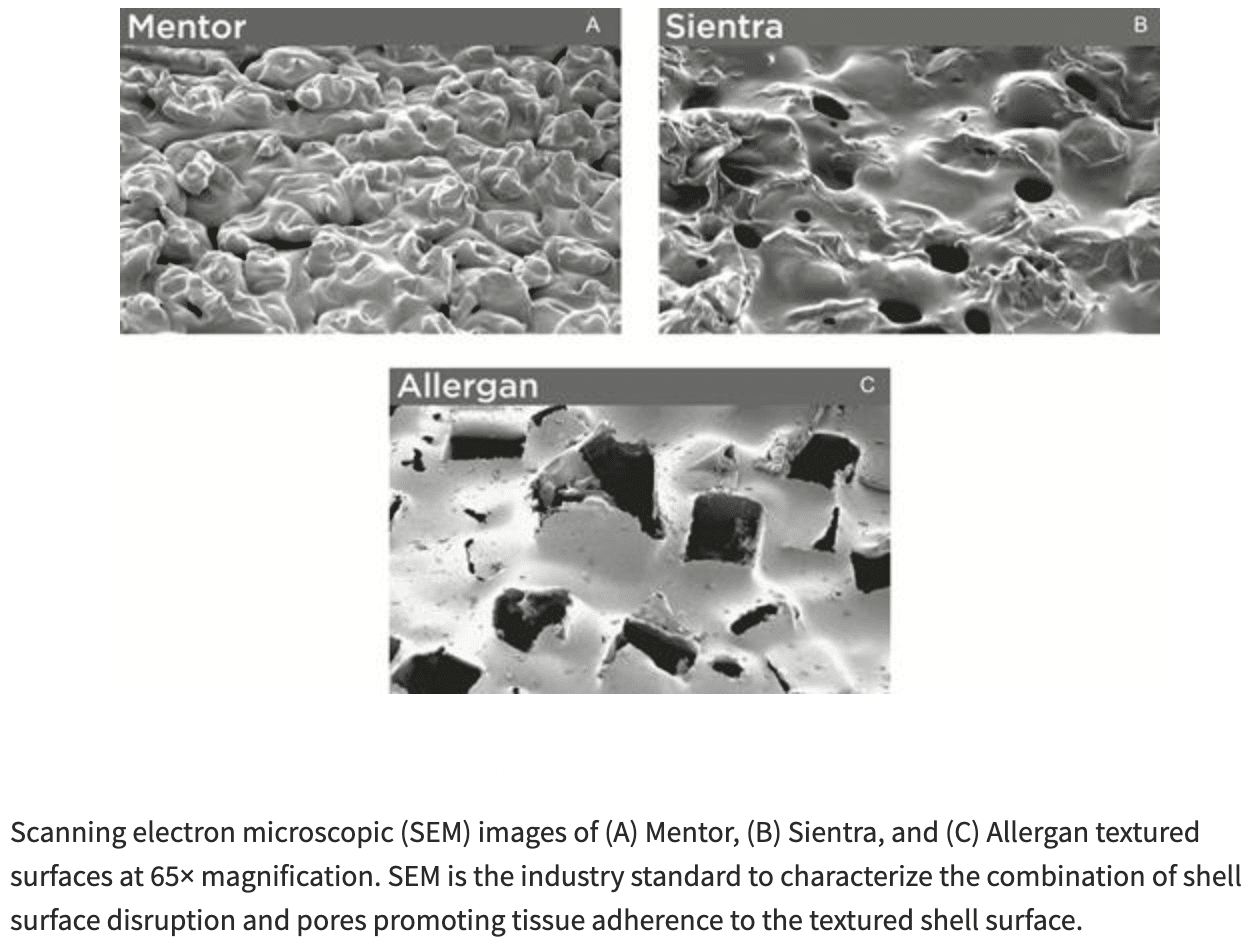
As you can see in the images below, each breast implant manufacturer uses a slightly different process to produce their texturing which results in different patterns and depths on the various implants. Breast implant texturing can be graded from grade 1 (smooth) to grade 4 (the most texture).
The aggressive surface of Allergan implants has been found to have six times greater risk for ALCL than other textured surfaces. About 84 percent of BIA-ALCL cases are attributed to Allergan implants, opposed to the other FDA-approved brands with texturing. Although all of the breast implant companies have all had at least 1 case of BIA-ALCL associated with their textured breast implants, Allergan’s textured implants specifically were removed from the market because they had a disproportionate number of cases of BIA-ALCL compared to the other implant manufacturers (Mentor worldwide and Sientra).
WHAT ARE THE CHANCES OF DEVELOPING BIA-ALCL?
A paper written by Dr. William P. Adams Jr. and myself that was published in the Journal of Aesthetic Surgery evaluated risk stratification using a concept called micromort. The value of one micromort is defined as a 1 in a million chance of death and is used to stratify the riskiness of day to day activities. To get an idea of this scale, simply getting out of bed as a 20-year-old increases risk of death by 1 micromort; driving a car for 1 hour per day increases risk of death by 2 micromorts; and running a marathon increases risk of death by 8 micromorts per run.
Using current statistics, the risk of a woman with textured breast implant dying from BIA-ALCL during her lifetime is about 1 micromort.
For comparison, the annual risk of a woman dying from breast cancer is about 500 micromorts. So although there is a risk for developing BIA-ALCL from textured breast implants, statistically speaking that risk is very small, or about 0.005%.
The FDA, along with all leading medical societies, does not recommend removal or replacement of textured breast implants in patients who have no symptoms. You are your best advocate for your breast health, so it’s important to know what the signs and symptoms of BIA-ALCL are and to pay attention to any changes in the breast implant size or shape of your breasts. If you have breast implants, continue to perform monthly self breast exams. If anything seems out of the ordinary, it is best to let your surgeon and/or primary care doctor know.
SYMPTOMS OF BIA-ALCL
The first symptom of developing BIA-ALCL is unexplained swelling, typically only seen in one breast. If you are experiencing breast swelling without any history of trauma to the breast, then now is a good time to contact your plastic surgeon. Other things to note:
- The average time to develop the condition is approximately 8-10 years post-implantation
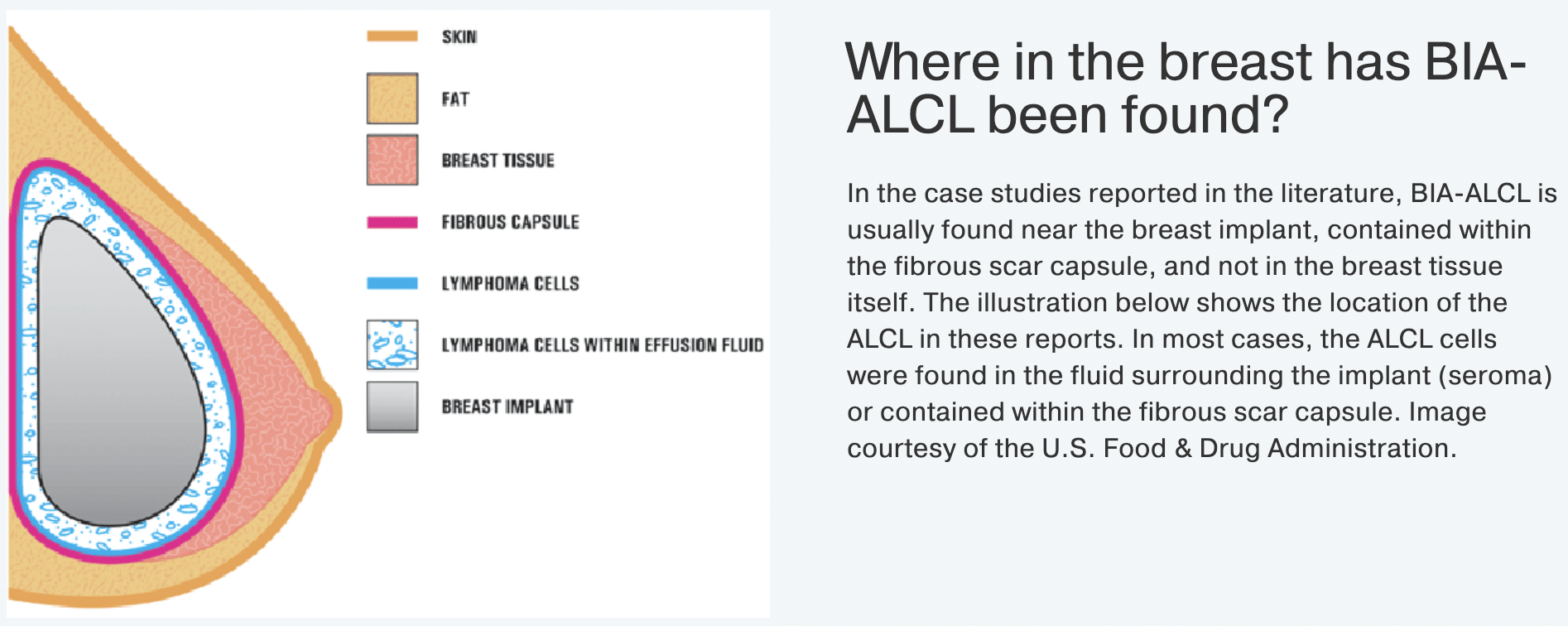
- BIA-ALCL most commonly shows up as a delayed seroma (collection of fluid) or a mass that can be felt, which develops years after the implants were placed
- This fluid can cause the breast to enlarge significantly over days or weeks. It can also present as a lump in the breast or armpit, firmness of the breast, or pain
If you suspect a change in your breasts, your surgeon will order an ultrasound or MRI to assess for any fluid buildup around the breast implant and collect the fluid for screening. Just like many skin cancers, when detected early, BIA-ALCL is a very treatable disease which can be successfully treated through surgery alone in the majority of cases. After the breast implants and surrounding capsule are removed, most patients won’t need any additional treatment. In all early stage cases, the disease was fully resolved by this surgery alone. For uninsured or underinsured patients diagnosed with BIA-ALCL, two separate funds have been established to help offset the costs for treatment. You can get more information and read about eligibility here.
I HAVE TEXTURED IMPLANTS – NOW WHAT?
If you don’t have any swelling and otherwise do not have any issues with your breast implants, then there is nothing else to do at this point other than continue monthly self breast exams. Currently, neither the FDA or any plastic surgery society recommends that women should remove textured breast implants to prevent BIA-ALCL. However, it is understandable that for some women, the concern about BIA-ALCL is enough to decide to have the textured implants removed prophylactically. If you’re reading this and don’t feel comfortable with the idea of living with your implants, then it’s a good idea to make an appointment with your plastic surgeon to discuss how to proceed.
OPTIONS AFTER BREAST IMPLANTS
Most women originally had a breast augmentation to increase their breast size or restore fullness to the breasts. If you still desire to have larger and/or fuller breasts, your surgeon can place new smooth silicone breast implants at the same time as the removal of your textured implants and capsules. As mentioned before, there have been no reported cases of developing BIA-ALCL due to smooth breast implants.
If you decide that you no longer want to have breast implants of any kind, then you can simply have them removed without replacement. However, if volume is still a concern, an option is to perform fat transfer to the breasts. This is done by performing liposuction on various parts of the body, then injecting the fat into the breast to improve breast size and shape without the need for implants. To be a candidate for this procedure, you do of course need to have fat somewhere on your body which can be harvested for transfer and understand that there are limitations to the size and shape that can be achieved through fat transfer alone.
If you have excess breast skin after your breast implants are removed, your San Francisco plastic surgeon may recommend a breast lift (mastopexy) to remove excess skin and reshape your breasts. At the same time fat may be transferred to the upper pole of the breast to add additional volume to help fill the areas previously occupied by a breast implant.
Questions and Answers
What are the issues with mentor implants?
Mentor has taken the proactive step of implementing a voluntary recall for particular batches of MENTOR® Smooth Round Saline Filled Diaphragm Valve (DV) Breast Implants with expiration dates ranging from January 01, 2025, to September 30, 2025.
What is the lawsuit against the mentor breast implants?
The lawsuit alleges that the Mentor breast implant’s faulty design and manufacturing processes were accountable for the emergence of cancer, contending that the company failed to adequately sterilize the implants prior to their distribution to hospitals.
Mentor Implants Recall: WHAT THIS ALL MEANS
If you have breast implants from a previous breast augmentation or mommy makeover procedure, you should determine if you have smooth or textured implants. If you currently have textured implants, realize that the risk of developing BIA-ALCL is very small and that the FDA and medical societies are not recommending the removal of breast implants in patients who have no symptoms. Continue to monitor for any changes in your breasts and know the early signs of BIA-ALCL (most frequently swelling on one side).
If you feel uneasy about keeping your breast implants, then see a board certified plastic surgeon to discuss your concerns and possible treatment options. For additional information on Allergan’s voluntary recall, including a list of affected products.
For in depth reading about BIA-ALCL, you can review the following links:
- Molecular Drivers of Breast-Implant Associated Anaplastic Large Cell Lymphoma (Blombery et al, 2019)
- Long-Term Safety of Textured and Smooth Breast Implants (Calobrace et al, 2018)
- 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) (Clemens et al; ASJ 2019):
- Current Risk Estimate of Breast Implant–Associated Anaplastic Large Cell Lymphoma in Textured Breast Implants (Collett et al, PRS 2019):
- What Cytokines Can Tell Us About the Pathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) (Kadin et al, ASJ 2019)
- Theories of Etiopathogeneis of Breast Implant-Associated Anaplastic Large Cell Lymphoma (Rastogi et al, PRS 2019)
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/allergan-voluntarily-recalls-biocellr-textured-breast-implants-and-tissue-expanders